2018 Volume No 35 – pages 73-86
Title: Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa |
Authors: A Dakiw Piaceski, D Larouche, K Ghani, F Bisson, S Cortez Ghio, S Larochelle, VJ Moulin, M Caruso, L Germain |
Address: Cancer Research Centre, Université Laval, Quebec City, Quebec, G1R 3S3, Canada |
E-mail: Manuel.Caruso at crchudequebec.ulaval.ca |
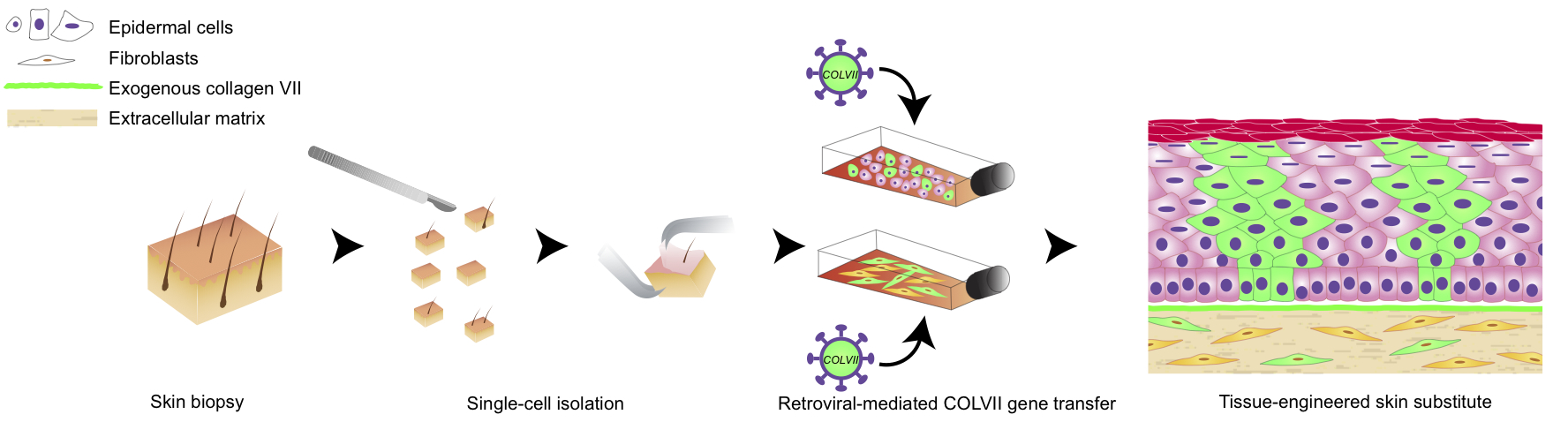
Abstract: The combination of gene therapy and tissue engineering is one of the most promising strategies for the treatment of recessive dystrophic epidermolysis bullosa (RDEB). RDEB is a rare genetic disease characterised by mutations in the COL7A1 gene, encoding type VII collagen (COLVII), which forms anchoring fibrils at the dermal-epidermal junction of the skin. This disease causes severe blistering and only palliative treatments are offered. In this study, the base of a strategy combining gene therapy and a tissue-engineered skin substitute (TES), which would be suitable for the permanent closure of skin wounds, was set-up. As a high transduction efficiency into fibroblasts and/or keratinocytes seems to be a prerequisite for a robust and sustained correction of RDEB, different envelope pseudotyped retroviral vectors and the transduction enhancer EF-C were tested. When green fluorescent protein (GFP) was used as a reporter gene to evaluate the retroviral-mediated gene transfer, the fibroblast infection efficiency was 30 % higher with the Ampho pseudotyped vector as compared with the other pseudotypes. At least a 3.1-fold and a 1.3-fold increased transduction were obtained in fibroblasts and keratinocytes, respectively, with EF-C as compared with polybrene. A continuous and intense deposit of haemagglutinin (HA)-COLVII was observed at the dermal-epidermal junction of self-assembled TESs made of cells transduced with a HA-tagged COL7A1 vector. Furthermore, HA-tagged basal epidermal cells expressing keratin 19 were observed in TESs, suggesting stem cell transduction. This approach could be a valuable therapeutic option to further develop, in order to improve the long-term life quality of RDEB patients. |
Key Words: Acantholysis bullosa, epidermolysis bullosa dystrophica, cell- and tissue-based therapy, tissue engineering, cell culture techniques, genetic therapy, collagen type VII. |
Publication date: February 14th 2018 |
Article download: Pages 73-86 (PDF file) |