2021 Volume No 41 – pages 401-420
Title: The role of altered glycosylation in human nucleus pulposus cells in inflammation and degeneration |
Authors: K Joyce, IL Mohd Isa, A Krouwels, L Creemers, A Devitt, A Pandit |
Address: CÚRAM, SFI Research Centre for Medical Devices, National University of Ireland, Galway, Ireland |
E-mail: abhay.pandit at nuigalway.ie |
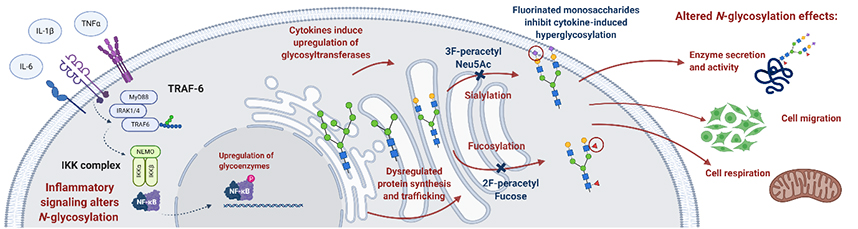
Abstract: Intervertebral disc (IVD) degeneration causes low-back pain through disc compression, prolapse and herniation. Inflammation of the IVD and subsequent degeneration produce altered glycosylation profiles in several animal models of IVD injury and ageing, although the function of this altered glycosylation pattern in a human is unknown. Altered N-glycome, specifically sialylated and fucosylated N-glycosylation motif expression, might play a role in inflammation and disease progression. Healthy (foetal and adolescent idiopathic scoliosis) and degenerated (lumbar degeneration) human IVD glycosylation patterns were studied using lectin histochemistry. Small-molecule fluorinated sugar analogues (3Fax-Peracetyl Neu5Ac; 2F-Peracetyl-Fucose) were used to inhibit sialylation and fucosylation in an in vitro model of inflammation, to investigate their effects on the glycosignature, cell metabolism, extracellular matrix synthesis and cell migration. The effects of interleukin (IL)-1β, tumour necrosis factor (TNF)-α and IL-6 on glycosylation in human nucleus pulposus cells were investigated by lectin histochemistry, PCR and enzyme-linked immunosorbent assay (ELISA). In the in vitro model of IVD degeneration, cytokine-induced inflammation-induced hypersialylation was observed, as indicated by Sambucus nigra I binding. However, this modification was inhibited by the sialyltransferase inhibitor. Inhibition of sialylation and fucosylation modulates cell migration and protein translation of catabolic enzymes in response to inflammation. The altered patterns of glycosylation in human tissue in degeneration was consistent with previous IVD studies in murine, bovine and ovine models. The present study was the first functional investigation of glycosylation in human degenerated IVD, elucidating the role of the glycome in disease progression and identified potential therapeutic targets for future regenerative therapies. |
Key Words: Glycosylation, inflammation, intervertebral disc, degeneration. |
Publication date: March 28th 2021 |
Article download: Pages
401-420 (PDF file) |