2022 Volume No 44 – pages 101-114
Title: FGF2 overrides key pro-fibrotic features of bone marrow stromal cells isolated from Modic type 1 change patients |
Authors: I Heggli, U Blache, N Herger, T Mengis, PK Jaeger, R Schuepbach, N Farshad-Amacker, F Brunner, JG Snedeker, M Farshad, O Distler, S Dudli |
Address: Balgrist Campus AG, Lengghalde 5, 8008 Zurich, Switzerland |
E-mail: irina.heggli at usz.ch |
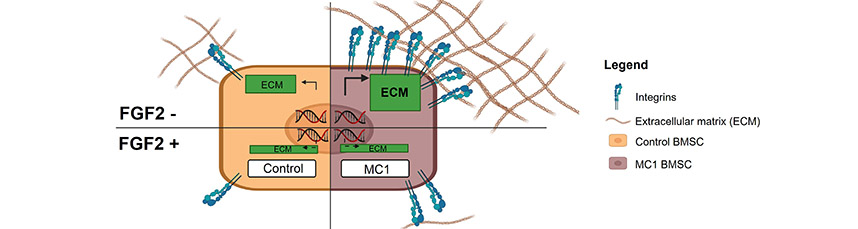
Abstract: Extensive extracellular matrix production and increased cell-matrix adhesion by bone marrow stromal cells
(BMSCs) are hallmarks of fibrotic alterations in the vertebral bone marrow known as Modic type 1 changes
(MC1). MC1 are associated with non-specific chronic low-back pain. To identify treatment targets for MC1,
in vitro studies using patient BMSCs are important to reveal pathological mechanisms. For the culture of
BMSCs, fibroblast growth factor 2 (FGF2) is widely used. However, FGF2 has been shown to suppress matrix
synthesis in various stromal cell populations. The aim of the present study was to investigate whether FGF2
affected the in vitro study of the fibrotic pathomechanisms of MC1-derived BMSCs. Transcriptomic changes
and changes in cell-matrix adhesion of MC1-derived BMSCs were compared to intra-patient control BMSCs
in response to FGF2. RNA sequencing and quantitative real-time polymerase chain reaction revealed that
pro-fibrotic genes and pathways were not detectable in MC1-derived BMSCs when cultured in the presence
of FGF2. In addition, significantly increased cell-matrix adhesion of MC1-derived BMSCs was abolished in
the presence of FGF2. In conclusion, the data demonstrated that FGF2 overrides key pro-fibrotic features
of MC1 BMSCs in vitro. Usage of FGF2-supplemented media in studies of fibrotic mechanisms should be
critically evaluated as it could override normally dominant biological and biophysical cues. |
Keywords: Bone marrow stromal cells, fibroblast growth factor 2, basic fibroblast growth factor, Modic changes, fibrosis, extracellular matrix, cell-matrix adhesion. |
Publication date: October 3rd 2022 |
Article download: Pages
101-114 (PDF file) |