2025 Volume No 52 – pages 98-113
Title: Seawater-contaminated ulnar rabbit bone defects repair using 3D-printed β-TCP/vancomycin composite scaffolds |
Authors: HD Lao, D Liu, R Yi, YN Yuan, M Zhao, GS Li, XY Nie, JL Gu, XM Cai, H Li, SE Lin, JJ Zhou |
Address: Stem Cells and Regenerative Medicine Laboratory, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Prince of Wales Hospital, 999077 Shatin, Hong Kong, China; Department of Orthopaedics & Traumatology, Musculoskeletal Research Laboratory, Faculty of Medicine, The Chinese University of Hong Kong, Prince of Wales Hospital, 999077 Shatin, Hong Kong, China; Department of Orthopaedics, 908th Hospital of Chinese People’s Liberation Army Joint Logistics Support Force (The Great Wall affiliated Hospital, Jiangxi Medical College, Nanchang University), 330001 Nanchang, Jiangxi, China |
E-mail: Sienlin at cuhk.edu.hk; Zjjortho at 163.com |
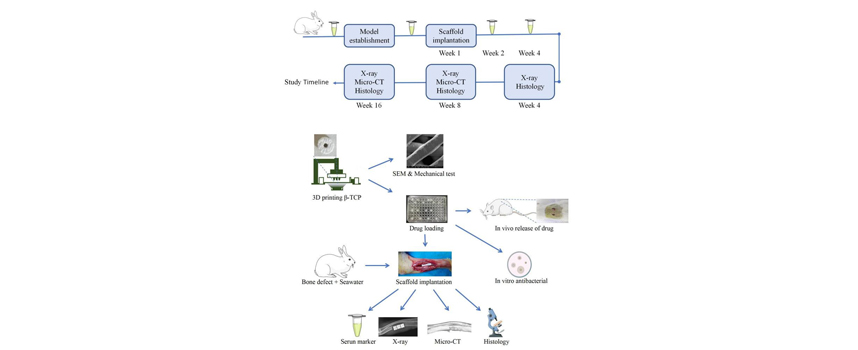
Abstract: Background: Repairing extensive bone defects following seawater immersion poses a significant challenge for orthopedic surgeons. Recent advancements in three-dimensional (3D) printing technology have demonstrated considerable potential in fabricating scaffolds with optimized morphological structures and superior biological properties. However, the specific characteristics and therapeutic efficacy of 3D-printed nano beta-tricalcium phosphate (β-TCP) scaffolds in the repair of seawater-immersed rabbit ulna bone defects remain inadequately explored. Methods: Nano-β-TCP scaffolds were fabricated via stereo lithography apparatus (SLA) and characterized using scanning electron microscope (SEM), X-ray diffraction, and mechanical testing. Vancomycin-loaded scaffolds were implanted in 18 rats, with drug release profiles monitored over a 56-day period. Thirty-six rabbits were assigned to three groups to assess scaffold performance in 1.5 cm seawater-immersed ulnar defects. Serum tumor necrosis factor-alpha (TNF-α) levels were measured pre- and post-implantation to evaluate inflammatory responses. Bone repair was assessed through X-ray, histological analysis, and micro-computed tomography (micro-CT) scanning. In vitro antibacterial efficacy was also evaluated. Results: The scaffold exhibited a cylindrical porous structure with dimensions of 0.5 cm in both diameter and height. The average pore size was approximately 400 µm, with a porosity of 53 %, and a compressive strength of 170 N. The scaffold demonstrated sustained vancomycin release over 56 days. In vivo, implantation of the scaffolds resulted in a significant reduction in serum TNF-α levels (p < 0.05) and promoted new bone formation compared to controls (p < 0.05). Histological and micro-CT analyses confirmed superior bone repair, with increased expression of osteocalcin (OCN), osteopontin (OPN), and vascular endothelial growth factor (VEGF). The scaffolds exhibited robust antibacterial activity after 72 hours. Conclusions: 3D-printed nano-β-TCP scaffolds offer an effective solution for repairing seawater-immersed bone defects and significantly enhance bone regeneration. |
Keywords: Materials science, biomedical materials, orthopedics, nanomaterials. |
Publication date: 28th August 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 98-113 (PDF file) |