2025 Volume No 53 – pages 15-27
Title: LGR4 binding small RANKL fragment inhibits RANKL-induced bone resorption |
Authors: Y Jang, YJ Cho, Y Ko, H Lee, B Kim, CM Lee, W Lim |
Address: Department of Premedical Program, School of Medicine, Chosun University, 61452 Gwangju, Republic of Korea |
E-mail: wonbong at chosun.ac.kr |
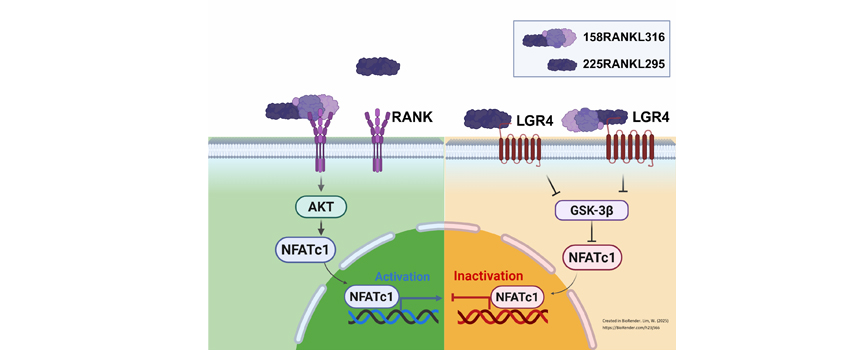
Abstract: Objective: Owing to the crucial role played by the osteoprotegerin-receptor activator of nuclear factor-kappa B-RANK ligand (OPG-RANK-RANKL) triad in osteoclastogenesis, the interaction among these proteins is an attractive target for developing osteoporosis drugs. RANKL binds to leucine-rich repeat containing G protein-coupled receptor 4 (LGR4) comparatively and suppresses the canonical signaling of RANK on osteoclast differentiation. In the present study, we investigated whether a RANKL-derived small peptide, selected based on the binding domain of RANKL with RANK, could lead the inhibition of osteoclast differentiation and activation in the absence of the RANK signal. Methods: We introduced small peptides into the RANKL protein based on their binding to LGR4 and investigated whether LGR4-RANKL signaling without RANK-RANKL could inhibit osteoclastogenesis. The binding affinities of RANK-peptides and LGR4-peptides were measured using microscale thermophoresis. The generation and activation of osteoclasts were assessed by tartrate-resistant acid phosphatase (TRAP) staining, bone resorption pit formation, real-time polymerase chain reaction, western blot, and nuclear translocation of nuclear factor of activated T cells (NFATc1) to determine RANKL-derived small peptide. Radiographic and histological analyses were performed to confirm the inhibitory effect on bone resorption in RANKL-induced mice. Results: Treatment with a RANKL-derived small peptide led to the binding of LGR4 without binding to RANK, as well as to the inhibition of NFATc1 nucleus translocation through the glycogen synthase kinase-3 beta (GSK-3β) signaling pathway, and showed an inhibition of TRAP activity and decrease of bone resorption in RANKL-induced mice. Conclusions: Our study provides a proof of concept that a small fragment of RANKL could be used as a novel therapeutic agent by inhibition of osteoclast through competitive inhibition of RANKL. This study suggests that RANKL-derived small peptides could be used as potential pharmaceutics for the osteoporosis treatment. |
Keywords: Bone resorption, TNF superfamily, osteoclast, osteoporosis, RANKL, LGR4. |
Publication date: 30th September 2025 |
Copyright policy: © 2025 The Author(s). Published by Forum Multimedia Publishing, LLC. This article is distributed in accordance with Creative Commons Attribution Licence (http://creativecommons.org/licenses/by/4.0/). |
Article download: Pages 15-27 (PDF file) |